Acridine ester fully automated chemiluminescence immunoassay reagent
Company Profile
Our mission is to provide high-quality products and services for human health
Beijing North Biotechnology Research Institute Co., Ltd. (hereinafter referred to as "North Biotechnology") was established in 1985 and is a wholly-owned subsidiary of China National Nuclear Corporation's CNNC Health Investment Co., Ltd. The company is committed to providing high-quality products and services for human health, and upholds the mission of focusing on in vitro diagnostics and serving human health. With 40 years of deep cultivation in the field of in vitro diagnostics (IVD), it is one of the earliest institutions in China engaged in the research and development, production, sales, and testing of immunological diagnostic reagents, and is a national high-tech enterprise.
Northern Biotech is closely following the "One Two Three" development strategy of CNNC Health, focusing on the in vitro diagnostic industry, establishing regional medical testing centers, creating commercial innovation platforms, and striving to become a well-known comprehensive service provider in the in vitro diagnostic industry in China. We have nearly 200 product registration certificates, and our production workshop has passed national GMP and ISO13485 certifications. We can produce more than 300 types of in vitro diagnostic reagents such as radioimmunoassay, enzyme-linked immunosorbent assay, colloidal gold, chemiluminescence, etc.
Our business covers multiple countries and regions around the world, and we have rich experience in production and operation. Our industrial development and expansion capabilities are sufficient. At the same time, the company actively expands its third-party medical testing business, with three independent third-party medical testing laboratories in Chengdu, Wuhan, and Hefei, and one diagnostic reagent production and research and development enterprise and one commercial innovation circulation platform in Shanghai. This lays a solid foundation for the company to build a fully segmented enterprise cluster that integrates the research and development, production, sales, and regional medical testing services of collective external diagnostic products.
Entering a new stage of development, Northern Biotech will aim to become a leading domestic enterprise in radiation immunology, a domestic alternative R&D platform for in vitro diagnostics, and a regional chain service provider for medical testing services. Keeping in mind its mission, we will break through innovation, continuously improve our independent R&D capabilities and quality control levels, and create a new highland for the development of the domestic in vitro diagnostics industry, contributing new strength to safeguarding people's lives and health!
year
The company was established in
+
Product Registration Certificate
+
Multiple in vitro diagnostic reagent products
family
Covering medical testing, diagnostic reagents, and commercial applications
Subsidiaries engaged in innovative circulation and other businesses

Corporate Culture
Corporate Purpose
Provide high-quality products and services for human health
Corporate Mission
Focusing on in vitro diagnostics to serve human health
Quality Culture
Cultivate quality genes, pursue technological progress, and achieve customer expectations with high-quality products
Development Goals
Focusing on the in vitro diagnostic industry, with scientific research and medical testing as the two wings, striving to build
To become a well-known comprehensive service provider in the in vitro diagnostic industry in China.
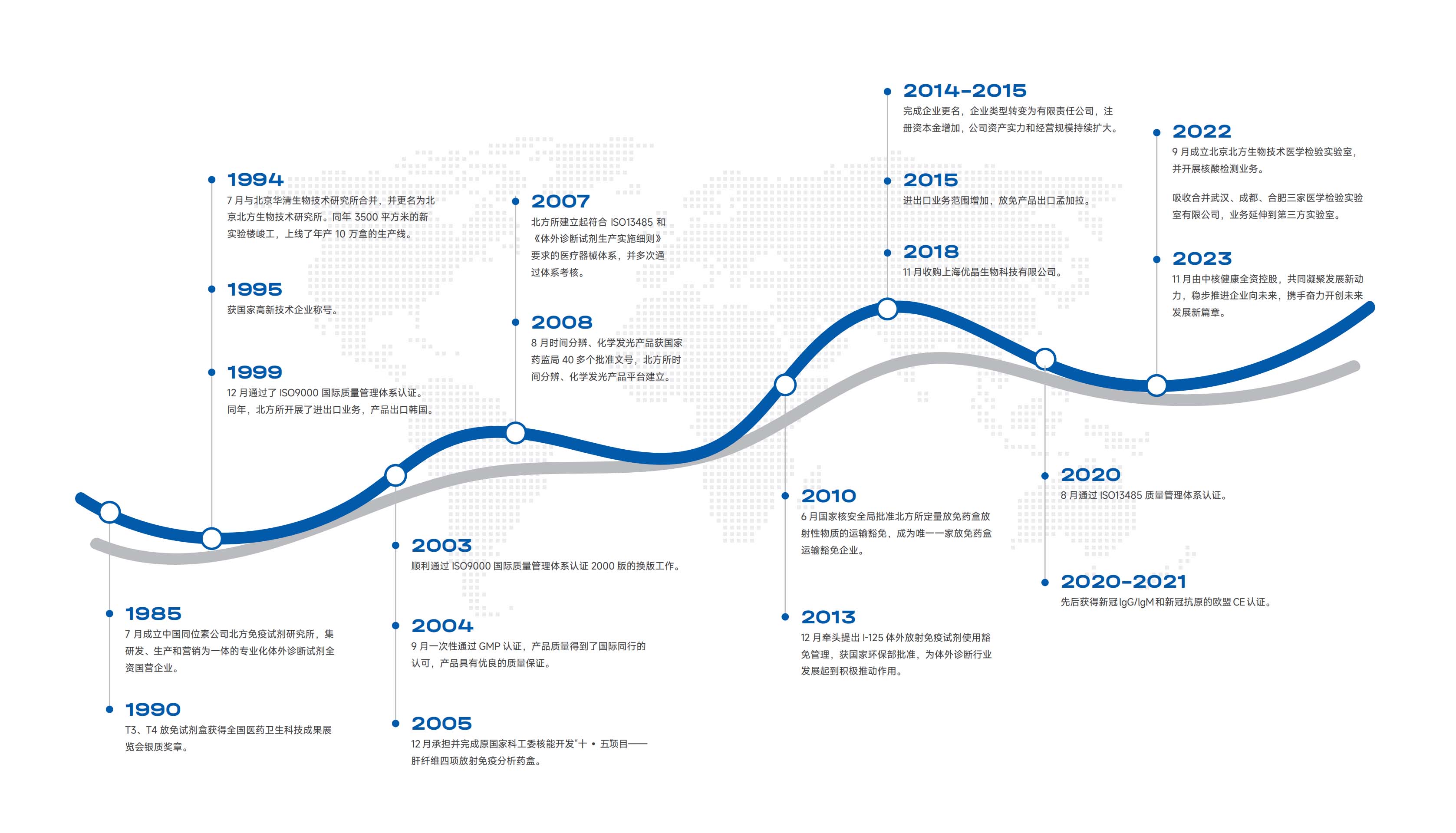
Development History